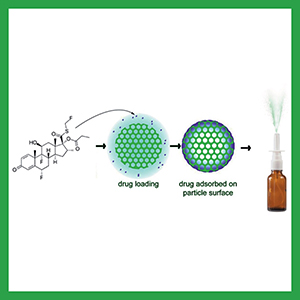
Efficacy of intranasal fluticasone propionate nano nasal spray in management of chronic rhinitis: a randomized clinical trial
Submitted: 8 November 2022
Accepted: 2 December 2022
Published: 30 December 2022
Accepted: 2 December 2022
Abstract Views: 1123
PDF: 366
HTML: 73
HTML: 73
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Yasir Mehmood, Hira Shahid, Aysha Tariq, Syeda Omama Ali, Efficacy and safety of a new mometasone furoate nasal spray formulation in patients with acute rhinosinusitis: a randomized clinical trial , Italian Journal of Medicine: Vol. 16 No. 1 (2022)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/itjm.2022.1545
https://doi.org/10.4081/itjm.2022.1545




